Using Xyloglucan Oligosaccharides as Biostimulant to Enhance Tobacco Tolerance to Salt Stress- Juniper Publishers
Journal of Agriculture Research- Juniper Publishers
Abstract
Xyloglucan oligosaccharides (XGOs) derived from the
hydrolysis of plant cell wall xyloglucan, are a new class of naturally
occurring biostimulants that exert a positive effect on plant growth and
morphology and can enhance plant’s tolerance to stress. Here, we aimed
to determine the influence of exogenous Tamarindus indica L. cell
wall-derived XGOs on Nicotiana tabacum’s tolerance to salt stress by
examining the plant’s morphology, physiological, and metabolic changes
after XGO application. N. tabacum plants were grown in solid media for
two months under salt stress with 100mM of sodium chloride (NaCl) ±
0.1μM XGO. Germination percentage (GP), number of leaves (NL), foliar
area (FA), primary root length (PRL), and density of lateral roots (DLR)
were measured. Also, 21-old-day N. tabacum plants were treated with a
salt shock (100mM NaCl) ± 0.1μM XGOs. Proline, total chlorophyll, and
total carbonyl contents in addition to lipid peroxidation degree and
activities of four enzymes related to oxidative stress were quantified.
Results showed that under saline conditions, XGOs caused a significant
increase in NL and PRL, promoted lateral root formation, produced an
increase in proline and total Chl contents, while reducing protein
oxidation and lipid peroxidation. Although they modulated the activity
of the enzymes analyzed, they were not statistically different from the
salt control. XGOs may act as metabolic inducers that trigger the
physiological responses for counteracting the negative effects of
oxidative stress under saline conditions.
Keywords: Antioxidant system; Biostimulants; Nicotiana tabacum; Salt stress; Xyloglucan oligosaccharides
Abbreviations: CAT:
Catalase; Chl: Chlorophyll; DLR: Density of Lateral Roots; FA: Foliar
Area; GP: Germination Percentage; GPX: Peroxidase; GR: Glutathione
Reductase; MS: Murashige and Skoog; NaCl: Sodium Chloride; NL: Number of
Leaves; PCA: Principal Component Analysis; PRL: Primary Root Length;
RL: Lateral Roots; ROS: Reactive Oxygen Species; SOD: Superoxide
Dismutase; XGOs: Xyloglucan Oligosaccharides
Introduction
Modern agriculture faces many challenges in order to
meet the growing demand for worldwide food. The world’s population is
growing at an accelerated rate. By the end of 2050 it is expected to
reach 9.8 billion people and 11.2 billion in 2100 according to the
“World Population Prospects: The 2017 Revision”, published by the United
Nations Department of Economic and Social Affairs. However, food
productivity and availability are decreasing as a result of the effects
of several biotic and abiotic factors. Therefore, several actions are
being taken to reduce these losses and to cope with the growing food
need for the world’s population.
Soil salinity is a worldwide phenomenon that occurs
under almost all climatic conditions and is a major impediment to
achieving increased crop yields. Using the FAO/UNESCO soil map of the
world (1970–1980), FAO estimated that 19.5% of irrigated land were
salt-affected soils, and of the almost 1.5 billion ha of dryland
agriculture, 32 million (2.1%) suffer from salinity problems [1].
Salt-affected soils are characterized by abundant quantities of neutral
soluble salts that adversely affect plant uptake of nutrients in the
soil and their growth [2]. Under salt stress, plants are also under
other types of stresses, which have deleterious effects on them such as
water stress, ionic toxicity, and nutritional deficiencies
[2]. Altogether, these conditions confer oxidative stress and
metabolic imbalance to plants [3]. Consequently, plants exposed
to high saline conditions shown growth inhibition or retardation.
The morphology of plants exposed to salinity can be affected
by soil salt concentrations, type of plant species, age, and plant
stages (vegetative or flowering), and/or the type of salt present
[4,5]. For example, there is a decrease in plant lengths, leaf (foliar)
areas, leaf numbers and root systems under high concentrations
of NaCl [4]. Also, many studies confirm the inhibitory effects of
salinity on photosynthesis by changing chlorophyll content thus
affecting Chl components and damaging the photosynthetic apparatus
[5].
In addition, plants exposed to high NaCl concentrations (such
as100-200mM) show rapid overproduction of reactive oxygen
species, which have detrimental effects on the plants’ cells. ROS
causes membrane lipid component peroxidation and oxidation of
cellular components such as proteins and nucleic acids, which finally
lead to programmed cell death [6,7]. ROS-initiated damage
is reduced and repaired by a complex antioxidant system, which
combines enzymatic and non-enzymatic components. It consists
of low molecular weight antioxidant metabolites, including ascorbic
acid, carotenoids, glutathione, α-tocopherol and enzymes such
as catalase, peroxidase, superoxide dismutase, glutathione reductase
and others. The degree of cellular damage will depend on the
balance between ROS production and elimination by the antioxidant
scavenging system [8].
Plants also accumulate compatible solutes in response to salt
stress, which provides protection to them by participating in ROS
detoxification and cellular osmotic regulation in addition to contributing
to enzyme/protein stabilization and membrane integrity
protection [6]. Among them, proline is one of the most important
ones due to its multiple roles as part of the plant’s response to
various types of stresses. It functions as an osmolyte for osmotic
adjustment, buffering cellular redox potential under stress conditions,
maintaining protein integrity, enhancing different enzymes
activities, and free radical scavenging [9,10]. Its accumulation in
leaves under salt stress has been correlated with stress tolerance
in many plant species, allowing them to survive under this type of
stress [6].
Many efforts have been done to overcome the problems associated
with high soil salinity and salt stress in plants. However,
the use of traditional physical and chemical methods for environmental
restoration of salt contaminated soils demand significant
investment of technological and economic resources [11]. In addition
to these traditional approaches, different biostimulant classes
have been used to increase crop performance under salt stress and
to mitigate stress-induced limitations [12-14]. A plant biostimulant
is any substance or microorganism that is applied to plants
with the aim of enhancing nutrition efficiency, abiotic stress tolerance
and/or crop quality traits regardless of its nutrients content.
By extension, they also designate commercial products containing
mixtures of such substances and/or microorganisms [15]. Plant
biostimulants based on natural materials have received considerable
attention by both the scientific community and commercial
enterprises. According to Stratistics Market Research Consulting
(MRC), the Global Biostimulants Market is accounted for $1.50
billion in 2016 and is expected to grow gradually to reach $3.79
billion by 2023 due to growing importance for organic products
in agricultural industries [16]. However, understanding the mechanisms
by which biostimulants act is critical to their widespread
use for helping plants cope in saline-affected soils.
XGOs, derived from the breakdown of xyloglucans in plant
cell walls, are emerging as a new class of naturally occurring biostimulants
as a result of their positive effects on plant growth and
morphology [17-19]. Plant-derived XGOs are also used as biotic
pesticides and seed coating agents to maintain plant freshness in
addition to capsule materials for synthetic seeds [20]. Xyloglucan
is the quantitatively predominant hemicellulosic polysaccharide
in the primary walls, which consists of ~20% (w/w) dicot and
~5% monocot primary cell walls [21]. Its backbone is composed
of a β-(1,4)-D-glucan backbone that is quasi-regularly substituted
with α-D-xylosyl residues linked to glucose through the O-6 position.
In many species, the backbone has a regular pattern of three
substituted glucose units followed by an unsubstituted glucose
residue [22]. As a variety of complex structures can be formed, a
code letter for each glucosyl residue has been defined to allow for
the unambiguous naming of xyloglucan oligosaccharides. For example,
XGOs can be classified as the XXXG-type of the XXGG-type,
in which a capital G represents a unbranched Glcp residue and a
capital F represents a Glcp residue that is substituted with a fucose-
containing trisaccharide [23]. Soluble XGOs can be obtained
from tamarind (Tamarindus indica L.) seeds after partial digestion
with cellulase. A fraction of these XGOs have been shown to have
physiologically active functions in plants and oligosaccharides,
also known as oligosaccharins [19,24]. Their biological properties
in plants depends on the fragmented structures and their concentrations,
which need to be extremely low to get a variety of effects
(10–9 - 10–8M) [17-19]. Few experimental data are available concerning
the use of the XGOs as plant biostimulants for mitigating
the damage imposed by salt stress conditions in plants [25]. Also,
each new formulation requires a new biological evaluation to ensure
that the effects are beneficial, consistent, and predictable.
For all of the above, the objective of this study was to determine
the biostimulating effects of application of exogenous XGO
derived from T. indica L. cell walls on N. tabacum seedlings grown
under saline stress conditions with special attention to their influence
on plant morphology, necessary physiological and metabolic
changes to overcome stress, ROS detoxification, and antioxidant
capacities.
Materials and Methods
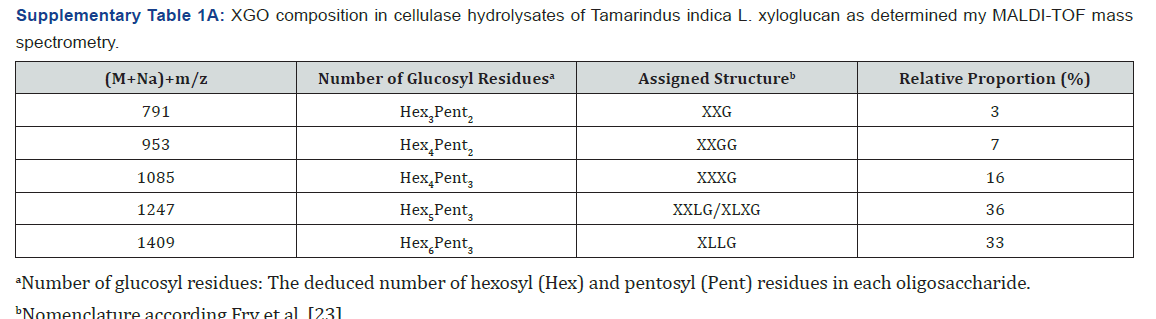
Oligosaccharin composition and concentration used
The XGO fraction used in this work was the same formulation
previously reported and tested on plants [18,26]. Briefly, XGO was extracted and purified from tamarind (T. indica L.) seeds.
The predominant composition of the XGO extracts consisted of
XLLG and XXLG/XLXG with lower proportions of XXXG, XXGG, and
XXG oligosaccharides as classified by Fry et al. [23]. Mass spectra
obtained by matrix-assisted laser desorption ionisation-time of
flight (MALDI-TOF) spectrometry [27] are shown in Supplementary
Table 1&1A. Relative proportions of xyloglucan oligosaccharides
obtained by MALDI and high-performance anion-exchange
chromatography with pulsed amperometric detection analysis
were similar (data not show). The isolated XGO fraction showed
no cellulase activity, and protein could not be detected. The uniformity
of the XGO mixture was confirmed by gel filtration analysis
through a BioGel P2 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). XGOs were used at a final concentration of 0.1μM which was
selected based on previous experiments as an optimal concentration
for stimulating root and leaf development in N. tabacum
without causing changes in plants’ chromosome number [17,28].
Plant material and general growth conditions
Botanical seeds of N. tabacum Linn. were used as the plant
model. Seeds were kindly provided by Dr. Alexis Acosta Maspons
from the Institute of Biotechnology of National Autonomous University
of Mexico (UNAM) (Cuernavaca, Morelos, Mexico). All
seeds were harvested at the same time, kept at 4 °C in the dark,
and grown under the same controlled conditions. Seeds were surface-
sterilized and grown on MS [29] solid medium in a growth
chamber (DAIHAN Scientific, model WISD, Korea) at 23 °C in longday
conditions (16h light/8h dark) and 50% relative humidity.
Sowing and the way in which each treatment was applied was
according to the chosen evaluation (plant and root morphology
measurements and biochemical analysis) and is explained in detail
for each one in this section. Each experiment was repeated to
generate three biological replicates.
Germination percentage and plant morphology measurements
Disinfected seed were sowed onto magenta boxes with MSagar-
media, either alone as a negative control, supplemented with
XGO at 0.1μM or 100mM NaCl to induce salt stress, or a combined
(both NaCl+XGO). The concentration of the NaCl solution was determined
based on experimental data (data not shown) in which
it was considerable biomass decrease in the presence of 100mM
NaCl was demonstrated. Each treatment consisted of nine seeds
per magenta box and five boxes per each biological replicate. Germination
percentage was calculated 10 days after sowing. Germination
criteria were considered complete germination after the
embryo emerged from the seed and a whole seedling was formed
[30]. For plant morphology analysis, seedlings were grown for two
months, and the number of leaves was then counted. Also, leaves
were harvested by cutting three of the oldest ones to measure their
foliar area, which were immediately photographed under a stereomicroscope
(Olympus, Model CX31-RTSF) coupled to an Infinity
Analyzer camera. FA was measured using the Infinity Analyze 3
software (Lumenera) according to the manufacturer instructions.
For root length measurements and lateral root primordium
frequency, sterilized seeds were plated on square Petri dishes in
a vertical orientation containing MS-agar-media as control or supplemented
with XGO at 0.1μM or 100mM NaCl to induce salt stress
or a combination of XGO+NaCl. Each treatment consisted of six
seeds per Petri square dishes and four dishes per each biological
replicate. All measurements were performed on 3-week old plants
that were fixed for 72h as previously described and had exhibited
whole root systems [18]. The total number of lateral roots, which
is the sum of the number of lateral root and number of primordia,
were counted directly under a stereomicroscope (Olympus, Model
CX31-RTSF). The fixed plants were then placed on a slide to allow
the primary root extension and measurements by taking photographs
and processing the images. A microscope (Olympus, Model
SZ2-ILTS) coupled to an Infinity Analyzer camera was used, and
primary roots lengths were measured using the Infinity Analyze 3
(Lumenera) software according to the manufacturer instructions.
Lateral root density (DLR, represented as D in the formula) per
mm of primary root length (PRL, represented as L in the formula)
was calculated using the following equation: D = (RL+P)/L, where
RL + P is the sum of the number of lateral root and number of
primordia [31].
Induction conditions for biochemical analysis
For all of the biochemical analyses, a uniform induction experiment
was designed in order to analyze XGO- (alone or in combination
with salt shock) induced dynamic changes in N. tabacum
seedlings. Specifically, we focused on the restitution phase (stage
of resistance, continuing stress) of plants’ stress response phases
[32]. Consequently, seeds were first placed in magenta boxes containing
MS-agar-media to allow their homogeneous growth for 21
days. After that time, four treatments were administered:
a. One mL of sterile distilled and deionized water as a negative
control.
b. A solution of 100 mM NaCl for salt shock.
c. 0.1μM XGO.
d. 0.1μM XGO+100mM NaCl were added to the top of the solid
media.
Sampling leaves were taken at two and five days after induction,
immediately frozen in liquid nitrogen, and stored at -80 °C
until their use.
Quantification of proline and photosynthetic pigment content
After two and five days of XGO application with or without salt
shock using NaCl 100mM, proline and total chlorophyll (Chl a+b)
contents were measured. Proline extractions and quantifications
were performed as previously described [33]. Briefly, the extract
was prepared by mixing 20mg of ground leaves in 1mL of 80%
ethanol, sonicated for 5min, and incubated for another 20min in
the dark. The mixture was centrifuged at 20,000g for 5min, and 200μL of the supernatant were added to 400μL of reaction mix
(ninhydrin 1% (w/v) in acetic acid 60 % (v/v), ethanol 20%
[v/v]), and heated at 95 °C for 20min. Finally, absorbance was determined
at 520nm using a FLU Ostar Omega Microplate Reader
(BMG LABTECH GmbH, Germany). A calibration curve was used
for proline concentration quantification and expressed as μg of
proline per mg of plant fresh weight. For photosynthetic pigment
content quantification, the Chl a and b and total chlorophyll (Chl
a+b) contents were extracted with 80 % acetone and measured as
described elsewhere [34].
Protein and lipid oxidative damage
Plant leaf tissue was ground to a fine powder with liquid nitrogen
to ensure sample homogenization. Protein oxidation was
measured using protein carbonyl content [35]. Briefly, 100μL
of sodium phosphate buffer (PBS, pH 7.8) was added to 400mg
of each sample, sonicated for 20min in the dark, centrifuged at
20,000g during 20min at 4 ºC, and the supernatant was used. Protein
concentration in the supernatant was measured using a BCA
Quantic Pro Sigma Kit to compare carbonyl content related to total
protein content. Total carbonyl content was measured using dinitrophenylhydrazine
(DNPH) reagent [35].
Lipid oxidation was analyzed with a thiobarbituric acid reactive
substances (TBARS) method [36]. For the assay, 200mg of
ground plant sample was submerged in 1000μL of acetone and
sonicated for 5min. The mixture was then incubated for 10min
in the dark and centrifuged at 4 °C and 20,000g for 5min. Then,
200μL of the supernatant was added to 300μL of a reaction mix
containing 2:1 of 20 % (v/v) trichloroacetic acid (TCA) and 0.67%
(w/v) of thiobarbituric acid (TBA). The reaction mix was heated at
95 °C for 15min, cooled at room temperature, and centrifuged at
20,000g at 4 °C for 20min. Lipid oxidation was measured by determining
the absorbance at 532nm using a FLU Ostar Omega Microplate
Reader (BMG LABTECH GmbH, Germany). The methylenedianiline
(MDA) standard and standard curve for the estimation
of total MDA were prepared as previously described [37]. Results
were indicated as A532 per gram of plant sample.
Antioxidant enzyme activities
Plant leaves collected (0.4g) were homogenized in liquid nitrogen
and 100μL of PBS (pH 7.8) containing protease inhibitor
(Sigma Aldrich) concentration 5X. The mixture was then sonicated
for 20 min in the dark and centrifuged at 20,000g at 4 ºC for
20min, after which time the protein content was measured using
a bicinchoninic acid (BCA) Sigma® kit. Enzyme activities were determined
immediately. Activities of the antioxidant enzymes, CAT
(EC EC 1.11.1.6), GPX (EC 1.11.1.7), GR (EC 1.6.4.2, GR), and SOD
(EC 1.15.1.1, SOD) were determined. The evaluation of enzymatic
activities was performed by comparing equal amounts of total
protein extracts from the samples collected.
CAT activity was measured by monitoring the enzyme-induced
decomposition of an H2O2 solution at 240nm and calculated
as H2O2 reduced per mg of protein per min [38]. GPX activity
was assayed as previously described [39], in which the reaction
mixture contained potassium phosphate buffer (100nM), guaiacol
(15mM, pH 6.5), H2O2 0.05 % (v/v), and 60μL of protein extract.
Guaiacol oxidation was monitored at 470nm and an enzyme unit
was defined as the production of 1μm of oxidized guaiacol per mg
of protein per min. SOD activity was measured by adapting the previously
described chromogenic assay [40] for leaf tissue protein
extract analysis. Briefly, for the reaction 225μL sodium pyrophosphate
(pH 8.3, 0.025M), 18.8μL (186μM) phenazine methosulfate,
56.3μL (300μM) nitroblue tetrazolium, 93.7μL distilled water, and
5μL of protein extract were mixed. To initiate the reaction, 37.5μL
(780μM) nicotinamide adenine dinucleotide was added, incubated
for 1.5 minutes and 187.5μL glacial acetic acid were added to
stop the reaction. The chromogen was extracted by addition of
700μL n-butanol followed by incubation for 10min, centrifugation
at 20,000g for 5min, and absorbance measurement at 560nm. For
GR activity measurement, the reaction was started by the addition
of oxidized glutathione, and the decrease in absorbance at 340nm
every min over a 3min period was read [41]. GR activity corresponded
to the amount of enzyme required to oxidize 1μmol min-1
of nicotinamide adenine dinucleotide phosphate. For all enzyme
activity analyses, results were expressed as U mg-1 protein.
Statistical analysis
For all variables analyzed, each experiment was performed in
triplicate. The data were expressed as average ± standard deviation
(SD) of the three independent replicates as a measure of dispersion.
For the variable NL, FA, PRL, and DLR, the data were evaluated
by an analysis of variance (ANOVA) by ranks (Kruskal Wallis
test) and compared using a nonparametric multiple comparison
test proposed by Conover [42] because the variables evaluated did
not show a normal distribution and had heterogeneous variances.
The adjustment to the premises was verified through the tests of
Shapiro Wilk and Levene. PCA was performed with Pearson correlation
matrices to represent a two-dimensional plane of treatment
effects upon the five morphological traits [43]. The values
of eigenvectors higher than the mean of the minor and the major
values of the component were considered as significant.
Data obtained from biochemical analyzes were processed
using a factorial ANOVA using a fixed effect model, in which the
factors consisted of the treatments (XGO±NaCl) and the days after
each treatment (two and five days). Previously, compliance with
the normality and homogeneity premises were verified through
the Shapiro-Wilk and Levene tests. All statistical analyses were
performed with the InfoStat program [44].
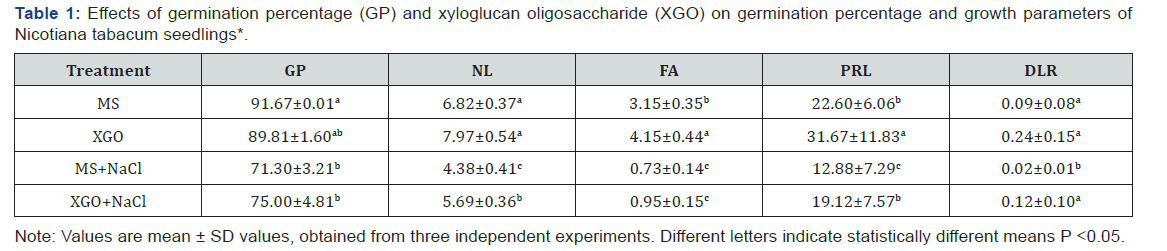
Resultst
Effect of XGO on germination and growth of Nicotiana
The effects on GP and plant and root growth of N.
tabacum
seedlings, grown with MS ± 0.1μM XGO, salt stress with ± 100mM
NaCl, or a combination of XGO and NaCl are shown in Table 1 and Figure
1. GP was statistically similar between the negative control
and 0.1μM of XGO (close to 90%). However, GP was significantly reduced
to 71% and 75% with 100 mM NaCl and 0.1μMXGO+100mM
NaCl, respectively, compared to the MS negative control (P<0.05).
Both salt stress control (MS+NaCl) and XGO+NaCl were statistically
similar. On the other hand, 0.1μM XGO significantly caused a
promotion in FA and PRL in two-month-old N. tabacum seedlings
compared to negative control, but no statistical differences were
observed in NL or in DLR. Moreover, when XGO was combined
with 100 mM NaCl, there was a significant increase in NL, PRL,
and DLR (P<0.05) related to salt control, but no statistical
differences
were observed in FA (Table 1 & Figure 1). Thus, addition of
100 mM NaCl caused an inhibition of NL and PRL by 36.3% and
43%, respectively, in N. tabacum seedlings compared to the MS
negative control. Nevertheless, the inhibitory effects of salt stress
on NL and PRL were reduced to 16.5% and 15.4%, respectively,
compared to untreated control when XGO was incorporated in the
media.
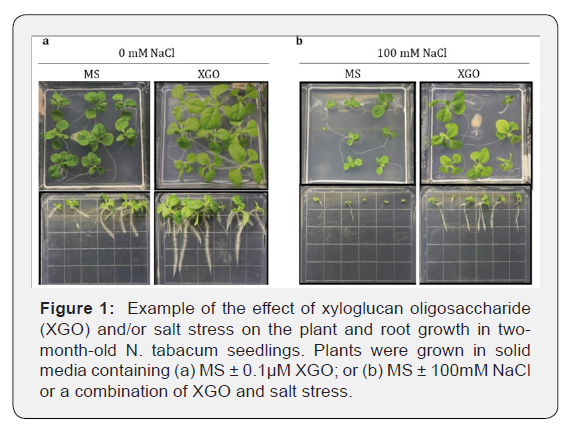
MS: Negative control; XGO: 0.1μM); MS+NaCl: Salt
stress with 100mM NaCl; XGO+NaCl: 0.1μM XGO + 100mM NaCl; GP:
Germination percentage;
NL: Number of leaves; FA: Foliar area (mm2); PRL: Primary root length
(mm); DLR: Density of lateral root.
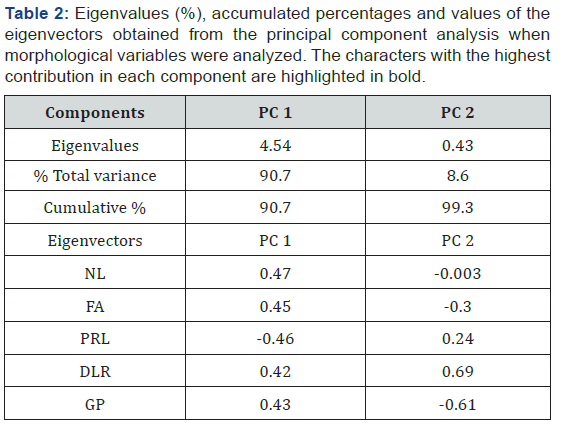
The PCA explained the contribution percentage of each component
to the total variation with the five quantitative characters
evaluated (GP, NL, FA, PRL, and DLR) and each treatment (XGO
and/or salt stress) (Table 2). The first two principal components
(PC 1 and 2) justified 99.3 % of the total variation. The characters
with the greatest contribution to variability consisted of NL, FA,
and PRL in PC 1, and DLR and GP in the PC 2. The biplot chart
of first and second component showed that PRL and DLR were
significantly correlated (P<0.05) in addition to NL, FA, PG, and NL
with PRL (Figure 2). There is a separation between treatments
with NaCl either with or without XGO application in the first principal
component, and in component 2 there was a clear separation
of XGO treatments from those without XGO. The higher values in
PRL, DLR, and NL correspond to treatments where XGO has applied
alone, compared to those where it was combined with salt
stress. In the second component, DLR and GP characters showed
the highest positive and negative contribution, respectively. Also,
there was a well-defined separation of XGO and XGO+NaCl from
MS and MS+NaCl, with the higher average DRL seen with the XGO
values.

Changes in proline and total chlorophyll content

Figure 3 shows the effect of XGO and salt stress on proline
and chlorophyll contents of N. tabacum leaves measured after two
and five days of induction. It can be noticed that treatments without
salt (MS±XGO) exhibited no significant differences in proline
content after two and five days of treatment (Figure 3a). Additionally,
salinity stress in N. tabacum seedlings promoted significant
proline accumulation compared to untreated control at both
time points. However, the results obtained XGO+NaCl application
reflects a gradual and significant increase in the proline content,
which is 75.98% higher than salt stress control after five days of
treatment (Figure 3a).
Total chlorophyll (Chl a+b) content was significantly higher
after XGO application compared to the untreated control, which
reached the highest levels among all treatments at both time
points (Figure 3b). Also, as expected the Chl a+b content was
significantly reduced by salinity stress in N. tabacum leaves compared
to the untreated control and remained constant over time
(P<0.05). On the other hand, XGO combined with NaCl produced
a significantly higher Chl a+b content (29.58% upper) compared
to salt stress control after five days of treatment, which reached
levels similar to the negative control.
Changes in protein and lipid oxidative damage
The effects of XGO and salt stress on protein oxidation, measured
as total carbonyl contents, in N. tabacum leaves at two and
five days after induction is shown in Figure 4a. Plants treated with
XGO exhibited no significant differences in total carbonyl content
related to control plants after two and five days of treatment. On
the other hand, NaCl application significantly increased its content
by 67.10% compared to the untreated control after five days. In
contrast, at the same time point, XGO application combined with
salt stress significantly reduced protein oxidation in N. tabacum
leaves by 98.45% compared to the salt stress control.
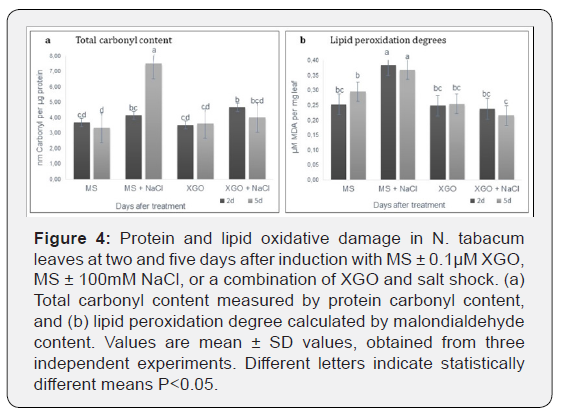
Lipid peroxidation was calculated in terms of MDA content
as an indicator of lipid oxidative deterioration caused by severe
oxidative stress in N. tabacum leaves at two and five days after induction
(Figure 4b). XGO application caused the MDA amounts to
remain constant over time. On the other hand, leaves from plants
exposed to salinity stress demonstrated significantly higher MDA
accumulation compared to untreated control at both time points.
However, XGO application and salt stress also significantly caused
a reduction in lipid peroxidation by 51.88% compared to salt
stress control after five days of treatment.
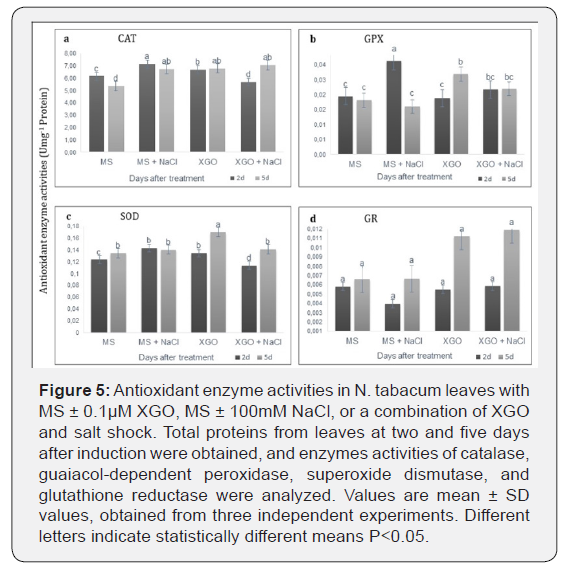
Changes in activities of enzymes from the antioxidant system
We also examined four oxidative stress response
enzyme
markers in the context of their activities. Figure 5 shows the effect
of XGO application and salt stress on the activities of the antioxidant
enzymes, CAT, GPX, SOD, and GR in N. tabacum leaves after
two and five days of induction. The results showed that five days
after treatment with XGO alone resulted in a significantly higher
CAT, GPX, and SOD activities over time compared to the negative
control (P <0.05) (Figure 5a-c). GR activity was not significantly
affected by the application of any treatment (Figure 5d). Salt shock
with 100mM NaCl induced significantly higher CAT, GPX, and SOD
activities two days after induction compared to the negative control.
After five days in the presence of NaCl, GPX decreased, but
CAT activity remained higher (P<0.05) than the untreated control.
There was also an apparent increase in CAT, GPX and SOD activities
after five days of treatment with XGO+NaCl, but their levels
were statistically similar to those of salt control (Figure 5a–c).
Discussion
This study provides evidence concerning the effects of exogenous
application of XGOs on enhancement of N. tabacum growth
and development under saline conditions and salt shock in order
to allow them to overcome the salt-stress limitations. Clearly, under
the conditions used in the current work, XGO alone had a positive
effect on NL, FA, and PRL although it was ineffective in promoting
DRL. However, combined with continuous salt stress, this
effect is rearranged, and XGO causes an increase in NL and PRL in
addition to promoting lateral root formation although no changes
in FA were observed compared to the salt control. Consequently,
in the presence of XGO the plants managed to recover from limitations
imposed by salt, so they display visible morphological characteristic
improvement (Figure 1).
The analysis of eigenvalues corresponding to morphological
changes is based on PCA analysis and explains >99% of the total
experimental variability within the two first components. This is
almost 100 % of the experimental variability that was achieved
by reducing up to two principal components. The five evaluated
traits showed a high contribution in one of the two first principal
components; thus, each trait was very important to explain the
variability observed in the experiment. PC 1, which explains the
highest percent of variability (90.7%) allowed for separation of
treatments under salt stress (100mM of NaCl) from those without
NaCl. The highest values of NL, PRL, and FA were obtained in the
medium with XGO and without salt stress, which were projected
in the positive quadrant of the component. This result clearly indicates
that XGO in the medium enhanced PLR and increased FA and
NL in tobacco seedlings. PC 2, which explains the rest of the variability
(8.6%), separates the treatments with XGO from the treatments
without this biostimulant. In this component, the traits that
showed the highest contribution were PG and DLR. According to
these findings, the presence of XGO in the medium stimulated
DLR, but the highest values of PG were obtained in the MS medium
without NaCl.
These results confirmed that external application of 0.1μM
XGO positively influenced plant growth and morphological features
even under salt stress conditions and could be correlated
with auxin-like activity. It is known that at approximately 1μM, at
least four different cellotetraose-based XGOs (XXXG, XXLG, XXFG,
and XLLG) mimic auxin by inducing growth [45]. In a previous
study, we confirmed that auxin-like activity of the same XGO fraction
mix and concentration used in this work on Arabidopsis thaliana
seedlings [18]. These are important findings since the ability
of a biostimulant to influence plant hormonal activity is one of
their many important benefits because they can exert large influences
that eventually will improve their health and growth. As
plant growth regulators plays an important role as chemical messengers,
they alert the plants when stressful environmental conditions
exist so they can initiate or increase their stress response
processes [46].
In this context, XGO may be acting as a “switches” that turn on
the plants for stressful situations by altering hormonal balances.
Zhang and Schmidt (1999) discuss the “switch” concept and give
some examples of other types of biostimulants that reinforce our
evidence. In this context, the results also suggested that exogenous
XGO applications could be acting as “pre-stress conditioners”
[46] and their effects are manifested by improving osmotic regulation,
photosynthetic efficiency, or by causing an increase in antioxidant
levels. This argument is based on the results in which it was
shown that 21-day-old plants exposed to external application of
0.1μM XGO had higher proline accumulation and total Chl content
in addition to higher CAT activity levels, GPX, and SOD compared
to untreated plants. Of more interest was their effect when applied
in combination with salt shock (XGO+NaCl) compared with those
treated with NaCl alone (MS+NaCl). In this case, the highest recorded
proline levels in addition to higher total Chl were observed
after five days of treatments. Enhancement of this antioxidant machinery
could be reflected in significant protein oxidation (total
carbonyl content) and lipid peroxidation reduction. Therefore, it
can be seen that the XGOs help the plant to cope with the effect of
saline stress via proline accumulation.
Osmotic regulation is an important mechanism for plant cellular
homeostasis under saline conditions in which proline is the
most common osmolyte for osmoprotection [47]. The higher accumulations of proline recorded with XGO and NaCl at five days after
treatment could be correlated with stress tolerance and may participate
in the stress signal influence on adaptive responses (Figure
2) [6]. Proline also contributes to stabilization of sub-cellular
structures (such as membranes and proteins), and its cytoplasmic
accumulation could help reduce oxidative stress-generated plant
protein and membrane damage after exposure to salinity [6]. This
effect can be inferred because of lower total carbonyl content levels
since protein-bound carbonyls represent a marker of global
protein oxidation and lipid peroxidation products as biomarkers
for oxidative stress that were observed in plants treated with
XGO+NaCl (Figure 3). Therefore, XGO indirectly helps the cell cope
with salt stress by maintaining cellular osmotic adjustment and
protein and lipid integrity.
Another important result indicated that XGO seems to mitigate
negative effects on photosynthesis in stressed plants by increasing
Chl content. This parameter was used because of salinity-
induced increase in chlorophyllase activity with the consequent
degradation of chlorophyll (at least transiently). Consequently, we
can deduce that the increase in exogenously XGO-induced total Chl
content enabled tobacco plants to tolerate salt-stress in addition
to promoting their development and growth. Similar results with
photosynthetic pigments were obtained in our lab when the same
XGO fraction mix was evaluated on A. thaliana seedling growth under
saline stress [48].
Regarding the activity of antioxidative enzymes, exogenous
application of 0.1μM XGO increased CAT, GPX and SOD enzyme
activities compared to the negative control after five days of treatment.
Due to the fact that no significant XGO+NaCl effects on enzymes’
activity that were analyzed in this work were observed, it
would be advisable to analyze other antioxidant enzymes to expand
the analysis in addition to determining its mode of action in
the enzymatic antioxidant system.
Our study revealed that XGO seem to be working as metabolic
inducers that trigger the physiological responses mentioned
above. Some results support that xyloglucan fragments do not
penetrate the cell, but instead, it has been suggested that the existence
of specific receptors on the plasma membrane, which interact
with the fragments, activate a signaling cascade inside the cell
[49]. However, to date, no specific candidate has been identified as
a possible receptor of these molecules [19]. Also, it has been suggested
that they can promote modifications or integrate into the
cell wall, which can affect not only the extracellular events in the
wall but also intracellular events [50]. These authors demonstrated
that incubation of pea stem segments partially bisected longitudinally
with a xyloglucan oligosaccharide (9mM XXXG), accelerated
the cell elongation by integration of xyloglucans as they were
incorporated into the cell wall and became transglycosylated by
xyloglucan endotransglycosylase (XET). According to this, XGO’s
effects observed in our work may also result from a signal transduction
XET-mediated or -induced cell wall modification cascade
but not from the oligosaccarides’ direct actions.
Conclusion
Overall, we conclude that XGO can exert beneficial impacts on
tobacco plants’ stress response either through hormone-like effects,
osmotic regulation, photosynthetic efficiency improvement,
and increase in antioxidant levels. They promote the proline accumulation
as an organic osmolyte and increase total chlorophyll
content and modify some antioxidative enzymes’ activities that
eventually affect development of plant roots’ growth and development
under salt stress. Further in vivo studies are needed to
confirm the antioxidant effect of XGOs during salt stress in crop
plants as well as to unravel their mechanism of action on oxidative
responses. However, according to these results, the exogenous application
of XGO as biostimulant at very low concentrations could
be considered an alternative for improving the growth and productivity
of crops of agronomic importance under salt stress.
To know more about Journal of Agriculture Research- https://juniperpublishers.com/artoaj/index.php
To know more about open access journal publishers click on Juniper publishers



Comments
Post a Comment