Assessment of Growth, Lipid Peroxidation and Reactive Oxygen Species Scavenging Capacity of Ten Elite Cassava Cultivars Subjected to Heat Stress-Juniper Publishers
Journal of Agriculture Research- Juniper Publishers
Cassava is an important source of energy-giving food
in the developing countries [1]. Cassava productivity is stable and
reliable, making the crop a candidate for reducing food insecurity,
hunger and poverty in developing countries [2]. Under normal growth
conditions, the crop gives high tuber yield and when the growth
conditions are sub-optimal, cassava tuber yield is satisfactory [1]. For
these reasons, cassava production in developing countries is expanding,
a situation that makes the crop suitable for meeting Sustainable
Development Goals (SDG) [2]. However, empirical evidence from climate
change studies suggested that most cassava production areas would
experience global warming and temperature extremes [3]. Indeed, heat
wave (high temperatures) has been reported during growing period of
cassava in Africa, Asia and Latin America [3]. As a warm temperate crop,
cassava has best
shoot and root growth and development at 25-32 ˚C [2]. Tempera
tures above the normal optimum are sensed as heat stress. Heat stress
upsets cellular equilibrium and lead to severe retardation of growth and
development, and even result in plant death [4]. One of the
physiological damages of oxidative stress caused by heat stress is lipid
peroxidation. Peroxidation results in the breakdown of lipids and
membrane functions by causing loss of fluidity, lipid cross-linking, and
inactivation of membrane enzymes [5]. The extent of lipid peroxidation
can be evaluated by measuring thiobarbituric acid reactive substances
(TBARS) content, which is a secondary breakdown product of lipid
peroxidation [6]. Hydrogen peroxide is the product of the first
detoxification process of superoxide radical by SOD before scavenging by
CAT and other peroxidases. Hydrogen peroxide production invariably
measures ROS scavenging ability of plants under heat stress.
Environmental stresses such as heat stress induce the accumulation of
proline in many plant species [4]. Proline plays a role in cellular
osmoregulation
and also exhibits many protective effects; plants with elevated
proline levels were reported to exhibit enhanced tolerance
to abiotic stresses [7]. Levels of proline can be increased either
by stimulation of its biosynthesis by 1-pyrroline-5-carboxylate
synthetase(s) (P5CS) or by inhibition of its degradation by proline
dehydrogenases [7].
Heat stress triggered an upsurge in production of reactive oxygen
species (ROS) such as superoxide radical (O2
−), singlet oxygen
(1O2), hydrogen peroxide (H2O2) and hydroxyl radicals (OH•) [8].
The ROS are produced from different sources in plants. Heat stress
causes ROS production in chloroplasts and mitochondria by disturbing
membrane stability and biochemical reactions such as the
activity of ribulose-1,5-bisphosphate carboxylase/oxygenase [9].
In addition, ROS are produced in mitochondria from membrane
instability, resulting in photorespiration and enzymes involved in
cellular respiration such as complex I and III in the mitochondrial
electron transport chain [10]. Furthermore, ROS are produced
from NADPH oxidases (NOX) in the plasma membrane, amine oxidase
in the apoplast and xanthine oxidase in peroxisomes, which
are all induced by environmental stimuli including heat [11,12].
Excessive production of ROS under heat stress damages plant
cells and tissues permanently by oxidation of cellular components
such as lipids, proteins and DNA [13]. To remove excessive ROS,
plants have developed detoxifying enzymes such as superoxide
dismutase (SOD), catalase (CAT), peroxidases (POX), glutathione
reductase (GR), ascorbate peroxidase (APX), and non-enzymatic
antioxidants such as ascorbate, glutathione, carotene and tocopherols
[13,14]. Apart from their destructive effects in cells, ROS can
also act as signaling molecules in many biological processes such
as stomatal closure, growth, development, and stress signaling
[15]. Due to this dual role of ROS, plants are able to fine-tune their
concentrations between certain thresholds by means of production
and scavenging mechanisms. Since this ROS homeostasis is
disrupted under stress in favour of production, constitutive and
induced enzymatic antioxidant defenses are considered a crucial
component of plant stress tolerance [8,14].
Physiological, antioxidant defence capacity and molecular
responses of cassava to drought stress have been reported [16-
18]. In the same vein, the antioxidant defence capacities of wheat
[19], rice [20], maize [20] have been investigated in response to
heat stress. However, responses and antioxidant defence capacity
of cassava to heat stress has not been reported. Equally, genetic
improvement of cassava for heat tolerance has not been given adequate
research attention. The objective of this study was to assess
growth, lipid peroxidation and reactive oxygen species scavenging
ability of ten commercial cultivars of cassava.
Materials and Methods
Planting materials and growth conditions
Stem cuttings of cassava cultivars TMS 4 (2) 1425, TMS
97/3200, TMS 91/02324, TMS 98/0505, TMS 98/0510 TME 419,
TME 12, UMUCASS 36, UMUCASS 37 and UMUCASS 38. were obtained
from the International Institute of Tropical Agriculture
(IITA), Ibadan. A stem cutting (10cm long), with more than two
nodes, was planted per plastic pot containing 8 kg sterilized sandy
loam soil with: рH оf 7.2 аnd саtiоn еxсhаngе сарасitу оf 15.3
сmоlkg-1. Daily, each plant was irrigated manually with 600mL to
water holding capacity bу tар wаtеr, рH 6.8. Plants were grown at
an average temperature of 26±2 ˚C under 65±5% relative humidity
and 7-9 hours of daylight before and after heat treatment.
Heat treatment and experimental design
Four weeks after planting, temperature was raised from 26 ˚C
and maintained at 40 ˚C for 30 minutes. The experimental design
was randomized complete-block in three replications. Fifteen uniform
plants were used per cultivar.
Measurement of growth parameters
At four weeks after planting and before heat treatment, number
of leaf, ѕhооt hеight, leaf аrеа, number of root and drу wеight
(biomass) of plants were dеtеrminеd. This was repeated four
weeks after the heat treatment and the differences recorded as
growth after exposure to heat stress. For drу wеight, plants were
carefully removed to obtain intact roots. Adhering soil particles on
roots were removed by dipping them in water before dried in аn
оvеn at 80 ˚C to a constant weight. Lеаf area was mеаѕurеd by a
leaf area meter.
Lеаf рrоlinе соntеnt
To examine the osmotic adjustment of plants, proline content
of the third fully expanded leaf from the top was determined according
to Bates et al. [21] 24 hours after heat treatment. Leaf tissues
(3g) were extracted in 2ml of sulphosalicylic acid. The same
volume of ninhydrin solution and glacial acetic acid was added.
The samples were heated at 100 ˚C for 10 minutes, cooled in an
ice bath and 5 ml of toluene was added. At 528 nm, absorbance by
toluene was measured.
Phеnоliсѕ соntеnt
The method of Julkunen-Titto [22] was used to determine leaf
total phenolics content 24 hours after heat treatment. Briefly, fresh
tissues (0.5g) of third fully expanded and matured leaves from
shoot tip were ground in 80% acetone and the homogenized mixture
collected. Thereafter, a mix of Folin-Ciocalteu reagent (1ml),
water (2ml) and the supernatant (0.1ml) were homogenized and
vigorously shook for 10 minutes. To the mix was added 5ml of Na-
2CO3 and the volume was brought to 10ml using distilled water.
Absorbance was read at 750nm wavelength.
Antioxidant enzyme assays
Enzyme activities were assayed from the fourth fully expanded
leaves from the shoot tip 24 hours after heat treatment. After
washing with distilled water, leaf sample (0.5g) was ground in cold
0.1mol/l phosphate buffer (pH 7.5) containing 0.5mmol/l EDTA.
The homogenized mixture was centrifuged at 4 ˚C for 15 minutes
at 15,000 x g. The supernatant served as enzyme assay in this
study.
Ascorbate peroxidase
Determination of activity of ascorbate peroxidase (APX) as
outlined by Nakano et al. [23] was followed. The 3ml-reaction
mixture contained 50mmol/l potassium phosphate (pH 7.0),
0.2mmol/l EDTA, 0.5mmol/l ascorbic acid, 2% H2O2 and 0.1ml of
enzyme extact. For one minute, a drop-in absorbance at 290 nm
was noted. Oxidation of ascorbate was calculated using the extinction
coefficient Ɛ =2.8/mmol/l/cm. One unit of APX activity was
defined as one mmol ascorbate oxidized /ml /min at 25 ˚C.
Superoxide dismutase
The method of Dhindsa and Dhindsa [24] was followed for
determination of activity of superoxide dismutase (SOD). In this
study, a unit of SOD was the enzyme extract that caused photo-reduction
of a half of inhibition of nitro-blue tetrazolium and SOD
activity expressed as unit/mg protein.
Catalase
Activity of catalase (CAT) was measured as described by Aebi
[25]. A 3ml-reaction mixture containing 0.1ml enzyme extract,
50mmol. /l phosphate buffer (pH 7.0 and 30mmol/l hydrogen
peroxide was conducted. Activity of CAT was determined by recording
absorbance of hydrogen peroxide at 240nm.
Peroxidase
The method of Hemeda and Klein [26] was used to determine
activity of реrоxidase (POD) in a reaction mixture that contained
enzyme extract, 0.05% guaiacol, 25mmol/l phosphate buffer (pH
7.0), 10mmol/l hydrogen proxide. The POD activity was determined
by absorbance at 470nm [ε = 26.6/(mmol/l cm).
Statistical analysis
A one-way analysis of variance was performed on data to determine
significance of the treatment effect using Statistical Analysis
Systems 9.1.3. At 5% level of probability, treatment means
were separated by Ducan’s Multiple Range Test.
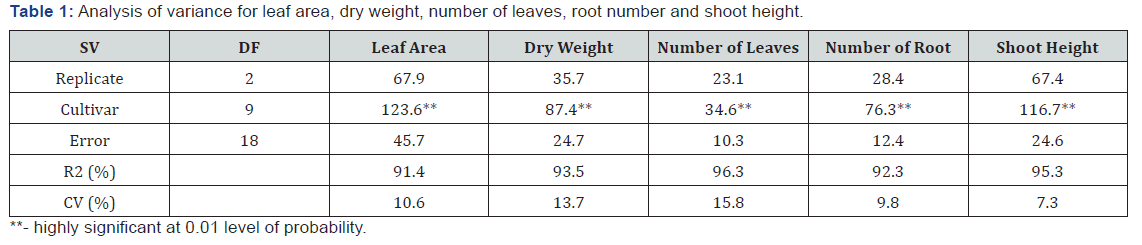
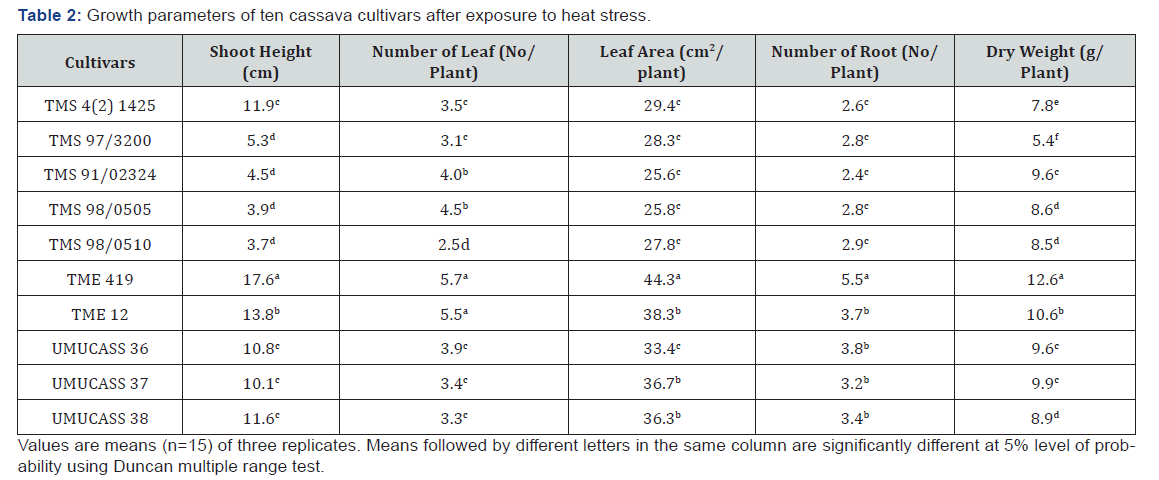
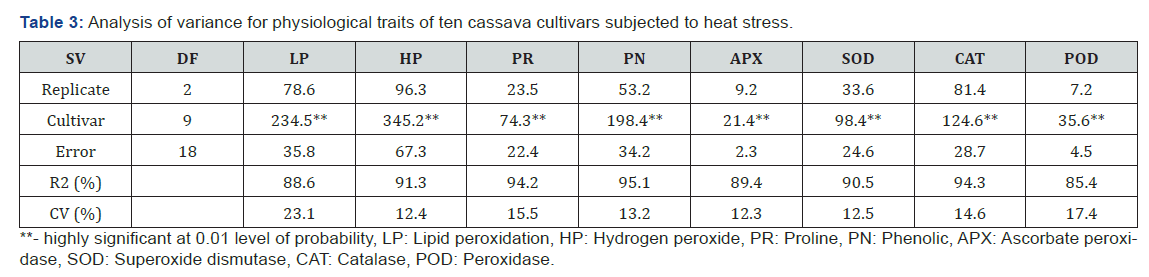
Results
Analysis of variance showed that cultivars differed for all
growth traits at 1% level of probability (Table 1). The R2 ranged
from 91.4 to 96.3%, while coefficient of variation ranged between
7.3 and 15.8%. All cultivars increased their shoot height, number
of leaf, leaf area, root number and dry weight after exposure to
heat stress (Table 2). Shoot height ranged from 17.6cm in TME
419 and 3.7cm in TMS 98/0510. The highest shoot (17.6cm) was
more than triple the least shoot heights (10.1-11.9cm) observed in
TMS 98/0510, TMS 98/0505, TMS 91/02324 and TMS 97/3200.
Leave production was ranged from 5.7 per plant in TME 419 and
TME 12 to 2.5 per plant in TMS 98/0510 (Table 2). Leaf area varied
from 44.3 to 25.6cm2/plant the highest leaf area was observed
in TME 419, followed by UMUCASS 37, UMUCASS 38 and TME 419.
The number of roots ranged from 5.5 per plant in TME 419 to 2.6
per plant in TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS
98/0505 and TMS 98/0510. Similarly, TME 419 recorded highest
(12.6g per plant) dry weight but TMS 97/3200 had the lowest (Table
2).
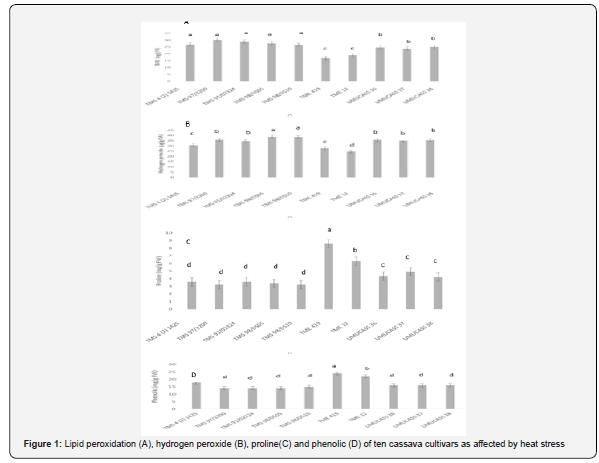
Analysis of variance showed that cultivars differed for all physiological
traits measured at 1% level of probability (Table 3). The
R2 ranged from 85.4 to 95.1%, while coefficient of variation ranged
between 12.3 and 23.1%. Lipid peroxidation ranged from 30.1 to
16.7mg/g of TBARS. The highest lipid peroxidation was observed
in TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS 98/0505
and TMS 98/0510 and least in TME 419 and TME 12 (Figure 1).
Hydrogen peroxide production ranged from 24.6 to 38.5μg/g.
Hydrogen peroxide production was highest in TMS 98/0505 and
TMS 98/0510 and lowest in TME 12. Among the cultivars, proline
content ranged 3.2 to 8.6mg/g while phenolic ranged from 14.0 to
24.0mg/g. While the highest proline and phenolic were produced
by TME 419, the lowest proline was recorded in TMS 4 (2) 1425,
TMS 97/3200, TMS 91/02324, TMS 98/0505 and TMS 98/0510
and lowest phenolic was found in TMS 97/3200, TMS 91/02324,
TMS 98/0505, TMS 98/0510, UMUCASS 36, UMUCASS 37 and
UMUCASS 38 (Figure 1).
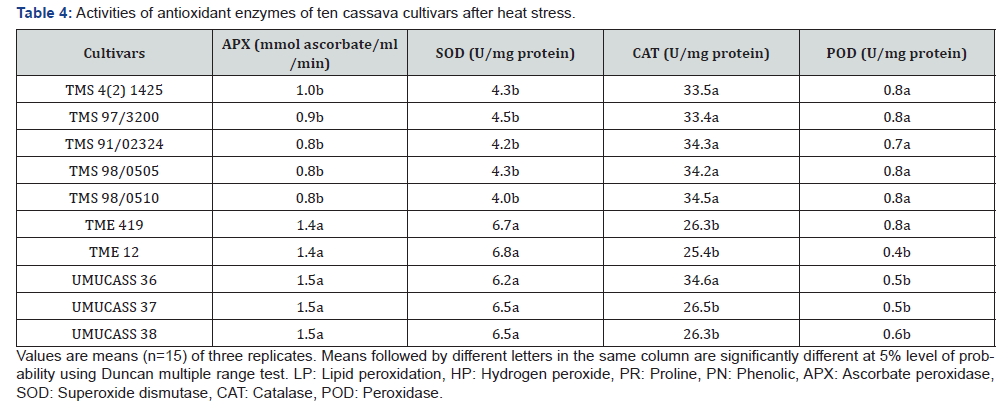
Activity of ascorbate peroxidase ranged between 0.8
and
1.5mmol ascorbate/ ml/min while the activity of superoxide dismutase
ranged between 4.0–6.7 unit/mg protein. TME 419, TME
12, UMUCASS 36, UMUCASS 37 and UMUCASS 38 had higher
ascorbate peroxidase and superoxide dismutase activities than
remaining cultivars (Table 4). In this study, catalase and peroxidase
activities ranged from 25.4-34.3 unit/mg protein and 0.4-0.8
unit/mg protein, respectively. However, catalase activity of TMS 4
(2) 1425, TMS 97/3200 TMS 91/02324 TMS 98/0505, UMUCASS
36 and TMS 98/0510 was higher than that of TME 419, TME 12,
UMUCASS 37 and UMUCASS 38. Cultivars were grouped into two
by peroxidase (POD) activity: the POD activity of the first group
(TMS 4 (2) 1425, TMS 97/3200, TMS 91/02324, TMS 98/0505,
TMS 98/0510 and TME 419) doubled POD activity of the second
group (TME 12, UMUCASS 36, UMUCASS 37 and UMUCASS 38, Table
4).
Discussion
Like other crops, cassava plants experience heat stress on the
field at all stages of its life cycle. Heat stress is exacerbated by climate
change and long growth cycle of cassava [2,3]. Heat stress
elicits molecular reactions in plants which triggers sequences of
physiological responses that manifested in morphological alterations
and adjustments [3]. In this study, it is noteworthy that exposure
of cassava young plants to high temperature (heat) did not
lead to total loss of growth in the ten cultivars investigated. Rather,
cultivars displayed varying degree of adjustments of morphological
traits as observed in shoot height, leaf area, root formation and
dry matter accumulation as allowed by their genetic constituent.
This implying that heat stress may not markedly reduce cassava
productivity of these popular cassava cultivars in Africa as the
plant may possess functioning heat tolerance mechanism. Variability
in growth responses among cassava cultivars observed in this
study agrees with previous report on morphological response to
heat stress wheat, maize and rice [19,20]. For instance, after heat
stress, cultivar 84-S had relative growth of 0.97g/g/day whereas
M-503 had relative growth of 0.101 g/g/day in cotton [27].
Plants have developed several heat stress adaptive responses.
In the present study, rapid increase in shoot height, to provide
certain physiological and metabolic advantages, could be heat
stress adaptive mechanism by TME 419 which is not present in
other cultivars. Adjustment in leaf formation is a vital stress-adaptive
mechanism in cassava to maintain metabolic processes. In
the present study, all cultivars continued production of leaves at
varying degree after exposure to heat stress indicating leaf formation
cessation was not caused by heat stress in the cultivars.
However, four cultivar displayed outstanding leaf production
under heat stress suggesting their tolerance to heat stress. Furthermore,
roots are essential organ of plants providing anchorage
and extracting water and nutrients for plants. All cultivars retain
their roots following exposure to heat stress indicating water and
nutrient absorption might not be severely disrupted under heat
stress in cassava. No plant was lost to heat stress. Dry matter accumulation
of most cultivars was impressive, suggesting cassava
has heat tolerance mechanism that allows dry matter production
under heat stress.
Our data suggested that heat stress caused damage to lipids
in cassava at varying magnitude across cultivars. Lipid oxidation
by reactive oxygen species has been established to be produced
by heat stress. For example, lipid peroxidation in cultivar 84-5 increased
by 79.9% by heat stress [27]. Limited lipid peroxidation
displayed in TME 419 and TME 12 could have resulted from low
quantity of ROS generated by the cultivars or destruction of ROS
by antioxidant enzymes. In addition, all cultivars produced hydrogen
peroxide, an ROS generated by heat stress indicating negative
metabolic machinery of the plants which must be removed to
prevent damage of proteins, lipids and DNA. Limited amount of
hydrogen peroxide observed in three cultivars (TMS 4 (2) 1425,
TME 419, TME 12) indicated that the cultivars have capacity to remove
hydrogen peroxide and thus tolerance of heat stress. While
heat stress increased hydrogen peroxide production by 50.0% in
drought-sensitive cultivar in cotton, heat stress has no effect on
hydrogen peroxide release drount-tolerant cultivar [27].
Proline (av. 5mg/g) was detected in all cultivars
after exposure
to heat stress. Gathering of proline to high concentration is one
of the early physiological reactions of plants experiencing abiotic
stress to amelioriate its negative effects. After six hours of heat
stress, high content (2.8-3.9pmol/g FW) of proline was observed in lower
leaves of wild and transgenic tobacco [28]. Drought-sensitive
cotton cultivar 84-S increased proline content by 5.9% after
exposure to heat stress [27]. We suggest that TME 419 and TME
12 that recorded outstanding quantity (6-7mg/g) of proline to be
exhibiting heat tolerance. Phenolics are produced by plants mainly
for protection against biotic and abiotic stresses. All cultivars
produced
high amount of phenolic suggesting that they were capable
of protecting themselves against adverse effects of heat stress.
Our results showed that APX, SOD, POD and CAT were active
in all cultivars subjected to heat stress in this study. Heat stress
has no effect on CAT activity in cotton [27]. Heat stress decreased
POD activity in cotton.by 41.3%. APX activity increased by heat
stress in some cultivar of cotton while heat stress had no effect on
APX in other cultivars of cotton. This is important because toxicity
of ROS to plants necessitated their immediate removal before destroying
cellular components [29]. Thе ROS аrе rеmоvеd bу these
аntiоxidаnt еnzуmеѕ which findings have suggested are involved
in stress tolerance in plants.
To know more about open access journal publishers click on Juniper publishers



Comments
Post a Comment